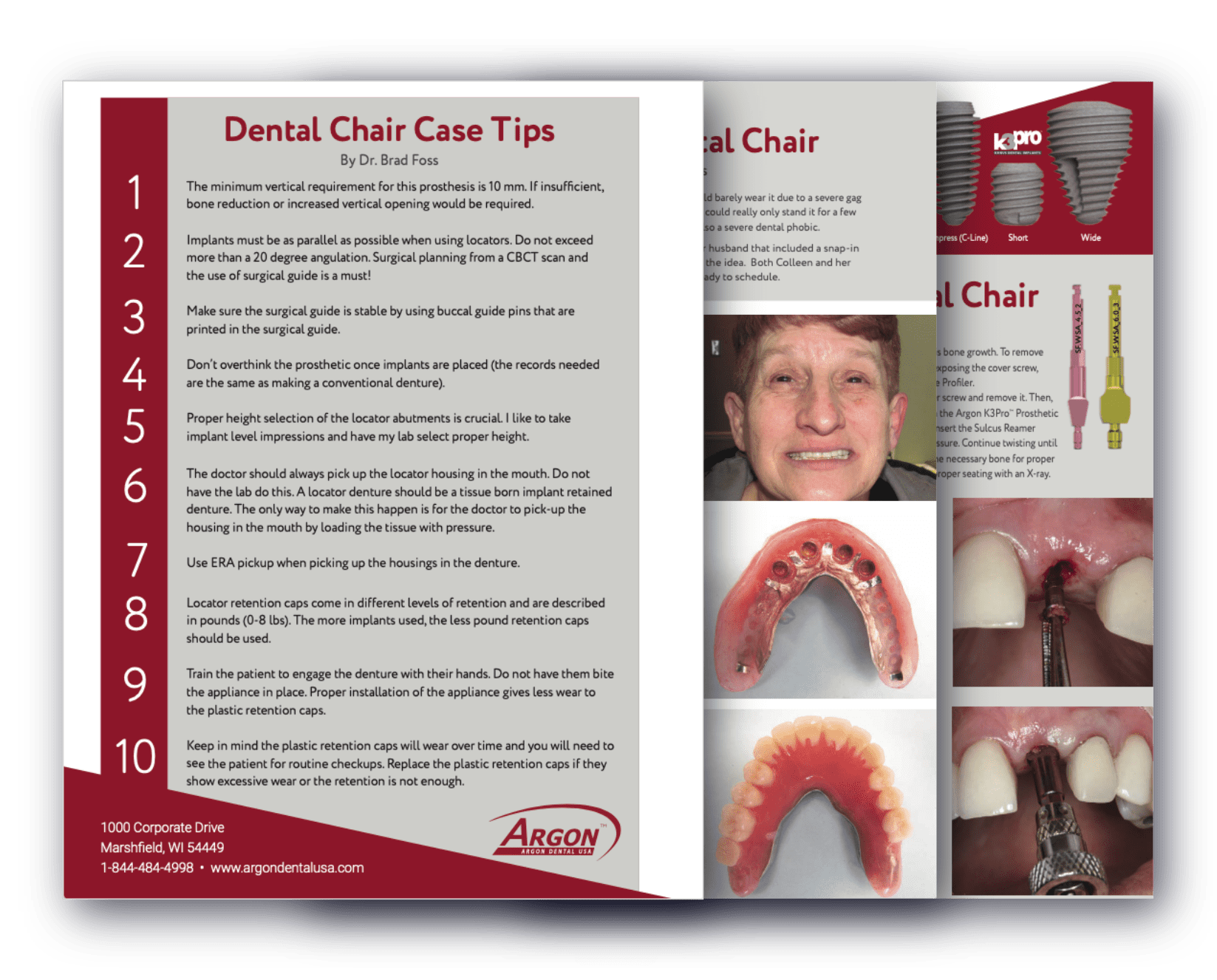
Osseointegration Guarantee

Limited Warranty
Argon provides solely to a Buyer that is also a treating clinician provider (“Clinician Buyer”) a limited warranty on the Products (“Limited Warranty” or the “Limited Warranty Program”). The Limited
Warranty is exclusively for the benefit of a Clinician Buyer and is not for the benefit of any other Buyer, including patients, laboratories, and other intermediate supplier. As such, there is no right, title, or interest in this Limited Warranty other than those expressly stated for a Clinician Buyer. For purposes of this Limited Warranty, the Clinician Buyer shall mean the individual that actually installed and purchased the Product. Products will be replaced if removal of said Product is due to the failure or defect of the Product itself, and if covered under the Limited Warranty set forth in this Section. The Limited Warranty shall only cover the costs of replacing the Product. Argon shall not cover any of the expenses included in the removal or installation of the Product. The total amount paid by or given in exchange for the Limited Warranty shall be limited and will not exceed the total amount the Clinician Buyer paid for the Product subject to the Limited Warranty claim. This Limited Warranty has no cash value and is not transferable to any third party.
Eligibility and Claim Procedure
To receive benefits under this Limited Warranty, the Clinician Buyer must comply with all of the following:
(a) a Limited Warranty claim must be reported to Argon within thirty (30) days from the date on which the claimed defect was discovered. Reporting shall fully comply with the procedure set out herein. Clinician
Buyer shall contact Argon Customer Service at 715-898-1434 or argon@argondentalusa.com or its Sales Representative to request a complaint record form and receive instructions for Product return; and
(b) the completed complaint record form, documenting the cause of the claimed failure, must be returned to Argon, accompanied by the Product in question, within the time stated above. Any Product must be
decontaminated prior to return to Argon. Clinician Buyers must mail the completed complaint record form and Product via certified mail return receipt requested to the attn of: Argon Dental USA. Failure to follow
shipping instructions shall void the Limited Warranty; and
(c) Clinician Buyers submitting a complaint record form for surgical benefits must provide documentation of the case and demonstration that Products were indicated and that no contra-indicated conditions existed
for that particular patient; and
(d) Clinician Buyers making a claim under this Limited Warranty must be current in all amounts owed to Argon at the time when the complaint record form is submitted; and
(e) all procedures using the Products – before, during and after implantation – must be performed in accordance with Argon protocols, guide lines and instructions, as well as generally accepted dental practices
Shipping
Transport costs and transport risk of the alleged defective Product shall be borne by the Clinician Buyer. In cases covered by this Limited Warranty, the cost of return shipment shall be borne by Argon.
Regulatory Requirements
Independent from Limited Warranty claims, complaints should be reported as soon as possible to comply with regulatory requirements.
Warranty Exclusions
(a) Argon shall not provide benefits under this Limited Warranty if:
(1) the Product failure is caused by a trauma, an accident, or by any other damage caused by the patient or a third party; or
(2) the Product failure is caused by a clinical component placed in patients with accepted contra-indicated conditions to successful implant integration, including but not limited to diseases related to
alcoholism, uncontrolled diabetes, and habitual drug dependency; or
(3) the Product failure is due to normal wear and tear; or
(4) the Product subject to the Limited Warranty claim is a single use instrument; or
(5) The Product subject to the Limited Warranty claim has been used in direct or indirect combination with a clinical component or instrument originating from another manufacturer than Argon’s
surgical instruments. Products consisting of surgical instruments should be replaced when they become worn, dull, corroded or in any way compromised. Surgical drills should be replaced after
20 to 25 osteotomies.1
Modification or Termination of the Limited Warranty
Argon reserves the right to modify or terminate its Limited Warranty Program in whole or in part at any time without notice. Such modification or the termination of the Limited
Warranty Program will not affect Products installed in patient, and fully paid by the Clinician Buyer to Argon, prior to the date of the modification or termination.
Disclaimer
EXCEPT FOR THE LIMITED WARRANTY SET FORTH HEREIN, ARGON MAKES NO WARRANTY WHATSOEVER WITH RESPECT TO THE PRODUCTS, INCLUDING ANY (A) WARRANTY OF
MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; (C) WARRANTY OF TITLE; OR (D) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A
THIRD PARTY; WHETHER EXPRESS OR IMPLIED BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
Third Party Products
Third Party Products are not covered by the Limited Warranty. For the avoidance of doubt, ARGON MAKES NO REPRESENTATIONS OR WARRANTIES WITH RESPECT TO ANY THIRD PARTY
PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; (C) WARRANTY OF TITLE; OR (D) WARRANTY AGAINST INFRINGEMENT
OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER EXPRESS OR IMPLIED BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE. Third Party
Products shall be subject to any Third Party Product warranty, if any, provided and offered directly by the manufacturer of such Third Party Product.
Exclusive Remedy
THE REMEDIES SET FORTH IN THIS AGREEMENT SHALL BE THE CLINICIAN BUYER’S SOLE AND EXCLUSIVE REMEDY AND ARGON’S ENTIRE LIABILITY FOR ANY BREACH OF THE
LIMITED WARRANTY. For the avoidance of doubt, this Limited Warranty Program, and the benefits and remedies set out herein, shall exclude any other rights, benefits and/or remedies, such as laboratory and clinical treatment related fees.
Limitation of Liability
IN NO EVENT SHALL ARGON BE LIABLE TO BUYER OR ANY THIRD PARTY FOR ANY LOSS OF USE, REVENUE, OR PROFIT, OR LOSS OF DATA, OR DIMINUTION IN VALUE, OR FOR ANY CONSEQUENTIAL,
INDIRECT, INCIDENTAL, SPECIAL, EXEMPLARY, OR PUNITIVE DAMAGES WHETHER ARISING OUT OF BREACH OF CONTRACT, TORT (INCLUDING NEGLIGENCE), OR OTHERWISE, REGARDLESS OF WHETHER SUCH DAMAGES WERE FORESEEABLE AND WHETHER OR NOT ARGON HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES, AND NOTWITHSTANDING THE FAILURE OF ANY AGREED OR OTHER REMEDY OF ITS ESSENTIAL PURPOSE.
To view our comprehensive Terms and Conditions, click here.
_____________________________________________________________________________________________________________________________________
1. Heat production by 3 implant drill systems after repeated drilling and sterilization. Chacon GE, Bower DL, Larsen PE, McGlumphy EA, Beck FM. J Oral Maxillofac Surg. 2006 Feb;64(2):265-9.